
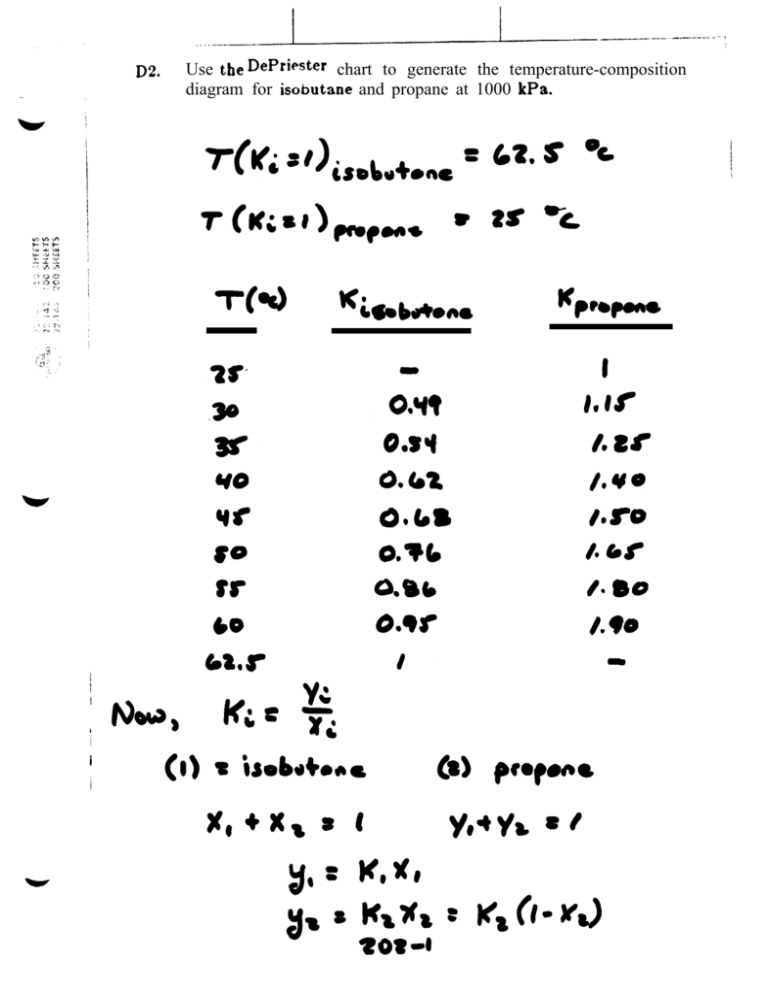
Note where the line crosses the methane axis.Connect the points with a straight line.On the right-hand vertical axis, locate and mark the point containing the temperature 60☏.On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia.Using DePriester charts, estimate the bubble point temperature of a mixture of 25(by mol) n-petane, 45 n-hexane, and 30 n-heptane mixture at 101.3 kpa. Example įor example, to find the K value of methane at 100 psia and 60 ☏. Using DePriester charts, estimate the bubble point temperature of a mixture of 25(by mol) n-petane, 45 n-hexane, and 30 n-heptane mixture at 101.3 kpa. Many DePriester charts have been printed for simple hydrocarbons. The K-value is where the green line intersects the curve for propane (K 0.8) Hess revenues increased to 1,919 million in the first quarter of 2021 from. The green line connects these two points. Use the sliders to set the pressure and temperature.
#HOW TO USE A DEPRIESTER CHART HOW TO#
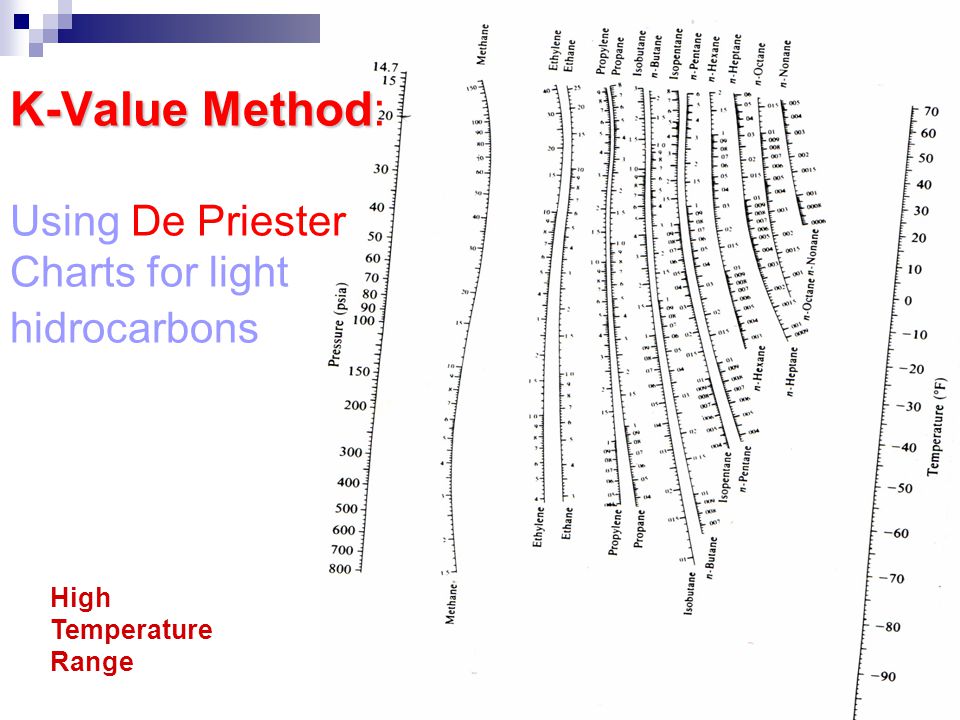
"K" values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between. Here is an example of how to use a DePriester chart for propane at 70 psia and 20 ☏: 1. These nomograms have two vertical coordinates, one for pressure, and another for temperature. DePriester in an article in Chemical Engineering Progress in 1953. Gauge pressure is depriester chart relative to the ambient pressure. Read this K-value off the chart (approximately 21.3).DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature. Separating the parts of a digit ISBN is also done with either hyphens or spaces, figuring out depriester chart to correctly separate a given ISBN number is complicated, because most of the parts do not use a fixed number of digits. Author: University of Colorado Boulder - Department of Chemical and Biological Engineering. (2) We can use K-values directly, by looking values up (DePriester Charts) or. The Pillars Curriculum for Chemical Engineering. Read this K-value off the chart approximately 21.
#HOW TO USE A DEPRIESTER CHART SERIES#
For mixture A shown in Table 2, a series of bubble pointand dewpoint calculations. View Notes - HW1 from CHEN 415 at American University of Beirut. Table 1 presents the list of suggested methods for estimation of K-values and the applicable pressure range. For light hydrocarbons, the approximate -values can be determined from DePriester charts, which have been fit to the following equation:, where the constants, ,, ,, and are tabulated 1. On the left-hand vertical axis, locate and mark the point containing the pressure 100 psia. In this Tip of the Month we will provide guidelines for use of these methods and present the results of a comparison study between these methods.Example įor example, to find the K value of methane at 100 psia and 60 ☏. "K" values, representing the tendency of a given chemical species to partition itself preferentially between liquid and vapor phases, are plotted in between. Vapor Pressure of Binary Solutions (Interactive Simulation) mirror. These nomograms have two vertical coordinates, one for pressure, and another for temperature. Using the DePriester Chart for Vapor Liquid Equilibrium (simulation) mirror. The -value is where the green line intersects the curve for propane (). DePriester in an article in Chemical Engineering Progress in 1953. Snapshots Details Here is an example of how to use a DePriester chart for propane at 70 psia and 20 ☏: 1. DePriester Charts provide an efficient method to find the vapor-liquid equilibrium ratios for different substances at different conditions of pressure and temperature.


 0 kommentar(er)
0 kommentar(er)
